Welcome to IDERHA training courses
IDERHA is one of the first research projects funded through the Innovative Health Initiative (IHI), a public-private partnership (PPP) between the European Union and the European life science industries that supports transformative research and innovation.
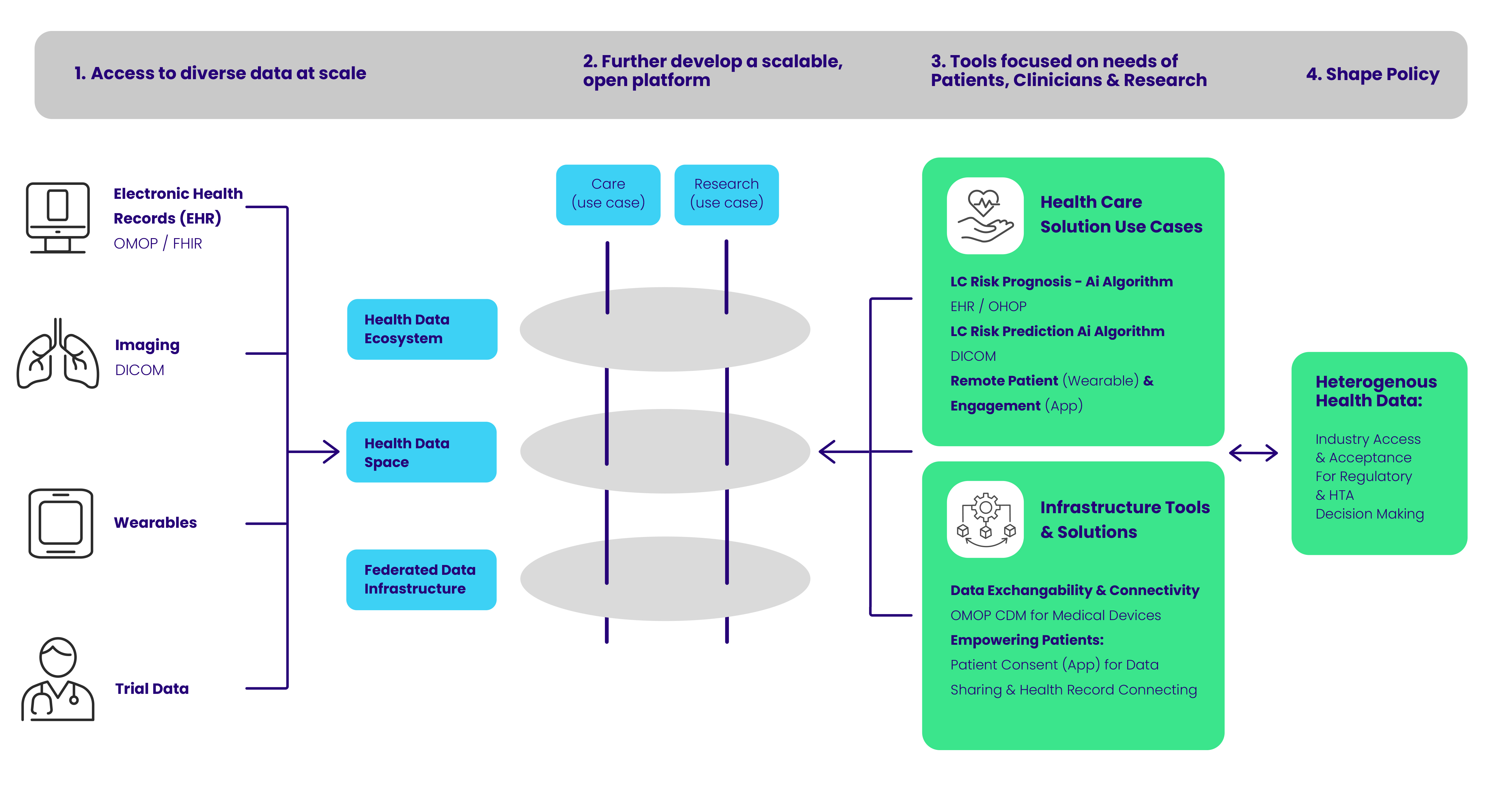
IDERHA aims to create a scalable platform for the seamless integration or linkage of diverse data at scale to support healthcare professionals, patients, and researchers with new capabilities to improve patient outcomes by better treating and manage disease, enable personalized care, bolster research and innovation, based on the development of common standards and practices – reducing current disconnected information silos. This enables more personalized health care along the patient's journey via data-driven tools and solutions.
This set of training courses indroduces basic skills about data sharing. In the picture below you see data sources like Electronic Health Records, Medical Imaging, Wearables and Trial data. This project aims to harmonize and standardize these data flows using existing international standards.
To learn more about these standards register for the courses listed below.
eHealth Standards and training materials
Discover the transformative world of IHE (Integrating the Healthcare Enterprise) with our comprehensive course. Dive deep into the evolution, significance, and practical applications of IHE in modern healthcare. Designed for both healthcare professionals and IT enthusiasts, this course offers a blend of theoretical knowledge and real-world insights. From understanding the history of IHE to exploring its pivotal role in healthcare interoperability, our modules are crafted to equip you with the expertise needed in today's digital healthcare landscape. Join us and embark on a journey to reshape patient care through seamless data integration and collaboration.
Visit our training course: https://moodle.technikum-wien.at/course/view.php?id=23419
Course registration
To register, please send an email to This email address is being protected from spambots. You need JavaScript enabled to view it. and provide first name, last name and email address! At the moment these courses are still in development and may be made accessible to members of the IDERHA consortium only.
Within IDERHA, the University of Applied Sciences Technikum Wien contributes its expertise in the areas of semantic interoperability, IT standards, APIs, data management and training. IDERHA is led by the Fraunhofer Institute for Translational Medicine and Pharmacology ITMP and Johnson & Johnson Medical GmbH, part of Johnson & Johnson MedTech, in a consortium of 33 academic, clinical, medtech, pharmaceutical, IT and patient advocacy organizations as well as public authorities. The project is scheduled to continue from April 2023 to March 2028.
Details and News can be found at https://www.iderha.org/
Training Materials Team
About IHI
The Innovative Health Initiative (IHI) aims to translate health research and innovation into real benefits for patients and society, and ensure that Europe remains at the cutting edge of interdisciplinary, sustainable, patient-centric health research. Health research and care increasingly involve diverse sectors. By supporting projects that bring these sectors together, IHI will pave the way for a more integrated approach to health care, covering prevention, diagnosis, treatment, and disease management.
IHI is a partnership between the European Union and European industry associations representing the pharmaceutical, medical technology, biotechnology, digital health and vaccine industries, namely COCIR, EFPIA, EuropaBio, MedTech Europe and Vaccines Europe. IHI’s total budget is EUR 2.4 billion. Half of this comes from Horizon Europe, the EU’s research and innovation programme. The IHI industry partners have committed EUR 1 billion to IHI, and a further EUR 200 million can be committed by other organisations that decide to become Contributing Partners.
IHI builds on the successes of the Innovative Medicines Initiative (IMI), and the IHI Programme Office continues to manage the IMI project portfolio.
Funding and disclaimer
This project is supported by the Innovative Health Initiative Joint Undertaking (JU) under grant agreement No 101112135. The JU receives support from the European Union’s Horizon Europe research and innovation programme and life science industries represented by COCIR, EFPIA / Vaccines Europe, EuropaBio and MedTech Europe.
IDERHA is funded by the European Union, the private members, and those contributing partners of the IHI JU. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the aforementioned parties. Neither of the aforementioned parties can be held responsible for them.








